Parts List
Friend
Find methods for your needs
AN190: Determination of Sulfate Counter Ion and Anionic Impurities in Aminoglycoside Drug Substances by Ion Chromatography (Impurities Method)
Description
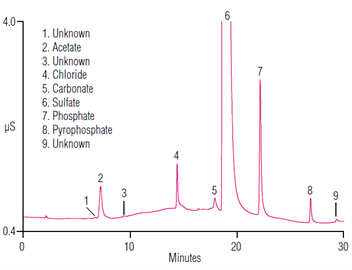
Many drug substances are in salt forms to promote solubility, stability, and bioavailability. It is important to accurately determine the concentration of the counter ions to establish the correct molecular mass, the stoichiometric relationship and the completeness of salt formation. This application note describes the linearity, detection limits, precisions, and recoveries using anion-exchange chromatography with an electrolytically-generated eluent. Here is the impurities method using IonPac AS11-HC column for the determination of sulfate counter ions and impurities in aminoglycosides.| Market: | Pharma |
| Keywords: | Acetate, Aminoglycoside, Anion-exchange, Anions, Chloride, Counter ion, Drug, Ion chromatography, IonPac AS11-HC, Pharmaceutical, Phosphate, Pyrophosphate, RFIC, Sulfate, Suppressed conductivity |
| Matrix: | Drug |
For Research Use Only. Not for use in diagnostic procedures.
