Find methods for your needs
Refine by Feature
Displaying 1-5 of 40 results for Tag: Pharmaceutical
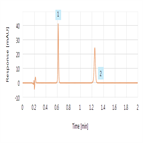
A rapid fenoprofen USP assay method
Instrument Type: UHPLCFive-fold increase in method throughput compared to original method (fifty samples/hour). Associated 10-fold reduction in cost per sample through reduced mobile phase consumption and waste generation. Additional reduced method complexity from easy-to-prepare mobile phase
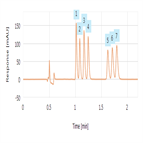
Simplified, high-throughput separation of glucocorticoids
Instrument Type: UHPLCImproved separation of closely related glucocorticoids with reduced method complexity High-throughput analysis possible through a reduced complexity, rapid, two-minute isocratic method Associated reduction in cost per sample through reduced mobile phase consumption and waste generation
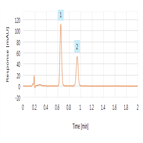
A rapid cephradine USP assay method
Instrument Type: UHPLCTo demonstrate practical approaches that can be used to significantly improve throughput of the cephradine USP assay monograph keeping to the spirit of USP-NF Chapter <621> guidelines while maintaining USP quality acceptance criteria.
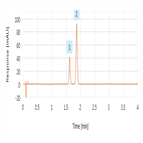
A rapid ibuprofen USP assay method
Instrument Type: UHPLCTo demonstrate practical approaches that can be used to significantly improve throughput of the ibuprofen USP assay monograph keeping to the spirit o USP-NF Chapter <621> guidelines while maintaining USP quality acceptance criteria. To then take this optimized assay monograph and reduce analysis time even further.
Quantitative and Semi-Quantitative Determination of PPCPs and Their By-Products in Wastewater by Orbitrap MS
Instrument Type: LCMSPharmaceuticals and personal care products (PPCPs) and endocrine disrupting chemicals (EDCs) detected in surface and drinking waters, as well as in treated wastewater. They are an issue of increasing international attention due to potential environmental impacts. We developed an analytical method capable of determining PPCPs and their by-products in wastewater treatment plant (WWTP) samples. This workflow was applied in a survey of 35 permeate samples obtained from a pilot anaerobic membrane bioreactor (AnMBR).