Parts List
Friend
Find methods for your needs
AN116: Quantification of Anions in Pharmaceuticals (Hydroxide Eluent)
Description
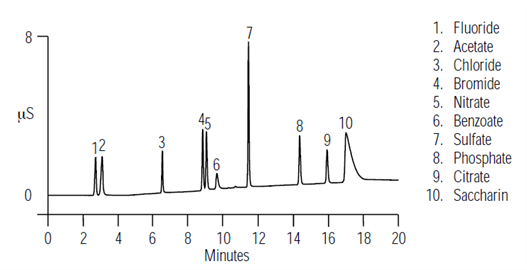
The United States Food and Drug Administration (U.S. FDA) and regulatory agencies in other countries require that pharmaceutical products be tested for composition. This Application Note describes the use of two anion exchange columns with suppressed conductivity detection to analyze common anions in pharmaceutical formulations. Here demonstrate that the IonPac AS11 resolves, in the same injection, common inorganic anions such as fluoride, chloride, bromide, sulfate, nitrate, and phosphate, as well as common organic anions such as benzoate, sorbate, citrate, and saccharin.| Market: | Pharma |
| Keywords: | Anions, benzoate, bromide, chloride, citrate, FDA, fluoride, Ion chromatography, nitrate, pharmaceutical, phosphate, saccharin, sorbate, sulfate, L61 |
| Matrix: | Drug |
For Research Use Only. Not for use in diagnostic procedures.
