Parts List
Friend
Find methods for your needs
AU167: Determination of Tobramycin in Crude and In-Process Production Samples During Manufacturing Using HPAE-IPAD
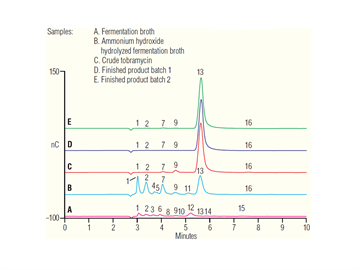
Description
This update describes how tobramycin and its typical impurities can be determined in manufacturing process matrices using the same HPAE-IPAD method described in AN 61. The same method is used to assay tobramycin that is intentionally degraded, demonstrating its suitability as a stability indicating method. An ICS-6000 can be used for this application.| Market: | BioPharma |
| Keywords: | Antibiotics, CarboPac PA1 column, HPAE-IPAD, IPAD, kanamycin B, nebramine, Dionex ICS-5000+ ion chromatography system, Fermentation broths, Tobramycin, Kanamycin A, Neamine (neomycin A), ICS-6000 |
| Matrix: | Fermentation broths |
| Author: | Valoran Hanko and Jeff Rohrer |
| Affiliation: | Thermo Fisher Scientific |
For Research Use Only. Not for use in diagnostic procedures.
