Find methods for your needs
Refine by Feature
Displaying 1-5 of 67 results for Tag: Antibiotic
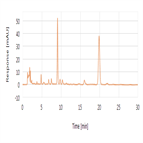
Composition and related substances analysis of colistin sulfate using a C18 HPLC column as per EP 8.4 monograph method
Instrument Type: HPLCThe Thermo Scientific Dionex UltiMate 3000 LC system is applied for the composition and related substances analysis of Colistin Sulfate. The analysis was performed on a Thermo Scientific Acclaim 120 C18 HPLC column using the method described in the EP-8.4 monograph. The results obtained for peak resolution met the criteria stated in the EP.
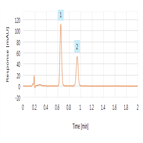
A rapid cephradine USP assay method
Instrument Type: UHPLCTo demonstrate practical approaches that can be used to significantly improve throughput of the cephradine USP assay monograph keeping to the spirit of USP-NF Chapter <621> guidelines while maintaining USP quality acceptance criteria.
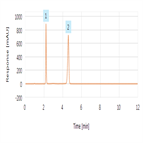
Assay analysis of Amoxicillin and Clavulanate Potassium as per USP
Instrument Type: HPLCThe Thermo Scientific Dionex UltiMate 3000 LC system is applied for the analysis of Amoxicillin and Clavulanate Assay as per USP. The analysis was performed on a Thermo Scientific Syncronis aQ L1 HPLC column using the method described in the USP 40 monograph. The results obtained were found to be within acceptance criteria as stated in the USP. Resolution between Amoxicillin and clavulanic acid was 14.0 (USP criteria: not less than 3.5). Tailing Factors observed were 0.84 & 1.25 respectively (USP criteria: not more than 1.5 for each analyte). RSD found to be less than 2% for both analytes
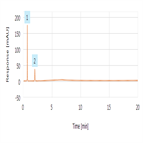
Related substances analysis of cefadroxyl monohydrate using a C18 HPLC column as per EP 8.0 monograph method
Instrument Type: HPLCThe Thermo Scientific Dionex UltiMate 3000 LC system is applied for the related substances analysis of cefadroxyl monohydrate. The analysis was performed on a Thermo Scientific Syncronis C18 HPLC column using the method described in the EP 8.0. The results obtained for resolution and signal-to-ratio met the criteria stated in the EP. The results obtained for resolution between the peaks due to EP Impurity A and EP Impurity B was 15.5 (EP criteria of minimum 5.0) and signal-to-ratio of EP Impurity B (0.4 µg/mL) was 56 (EP criteria of minimum 10). A sample chromatography is attached.
Trace analysis of pharmaceuticals and organic contaminants in water
Instrument Type: LCMSMSWe present the reliable and accurate quantitative analysis of contaminants at the pg/mL level in drinking water using the Thermo Scientific EQuan MAX Plus LC-MS system coupled to the Thermo Scientific TSQ Endura triple quadrupole mass spectrometer. Excellent reproducibility was shown for the target compounds in tap water using 1 mL injections at 0.37× maximum effluent concentration.