Find methods for your needs
Refine by Feature
Displaying 1-5 of 176 results for Tag: BioPharma
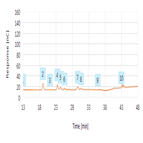
High-precision, automated peptide mapping of proteins
Instrument Type: UHPLCThe biopharmaceutical industry continues to develop protein-based biotherapeutics in increasing numbers. Due to their complexity and biotechnological production, there are many attributes that need to be analyzed to guarantee their safety and efficacy. Peptide mapping is used to measure several critical quality attributes (CQA) required for the characterization of any biotherapeutic protein. The analysis is used to confirm that the correct sequence has been expressed for the protein and to check for post-translational and chemical modifications.
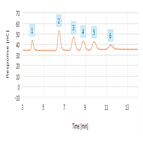
TN72264: HPAE-PAD N-linked Oligosaccharide Profiling of IgG
Instrument Type: ICThis technical note reports an HPAE-PAD method to profile IgG N-linked oligosaccharides and N-linked high-mannose type oligosaccharides that are atypical for human polyclonal IgG but sometimes found on mAbs. This HPAE-PAD method is orthogonal to other oligosaccharide methods applied to IgG. More information on the development and application of the separation described in the technical note can be found in Rohrer, et al. as referenced in the document.
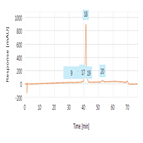
Separation of oxidized and deamidated impurities of Infliximab by reverse phase chromatography
Instrument Type: HPLC (Biocompatible)The Thermo Scientific Dionex UltiMate 3000 Bio-LC system is applied for the Reverse phase analysis of Infliximab. The separation was performed on a Thermo Scientific MabPac RP column with UV detection at 214 nm. All oxidized and deamidated impurities are well separated.
TN72225: Glycoprotein Monosaccharide Analysis using HPAE-PAD with Manually Prepared Eluent
Instrument Type: ICThis work describes an HPAE-PAD method for monosaccharide composition analysis using manually prepared eluent. Monosaccharide analysis using electrolytically generated eluent has been described before. Here, three commercially available proteins, IgG, fetuin, and alpha-1-acid glycoprotein, were individually subjected to two hydrolysis conditions using 1)HCl, for the amino sugars galactosamine and glucosamine, and 2)TFA, for the neutral sugars mannose, glucose, and galactose. Results for method linearity, robustness, and accuracy are presented here. An ICS-6000 can be used for this application.
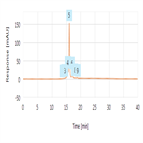
High resolution charge variants analysis of recombinant human IgG1K NIST mAb
Instrument Type: HPLC-CADThe Thermo Scientific Vanquish Flex UHPLC system with UV detection is applied for the charge variants analysis of NISTmAb Humanized IgG1K monoclonal antibody using strong cation exchange-based chromatographic separation on a MAbPac SCX-10 column. The use of Thermo Scientific CX-1 pH gradient buffers enabled high resolution separation of reference material monoclonal antibody charge variants using a 30 minute gradient.