Parts List
Friend
Find methods for your needs
Description
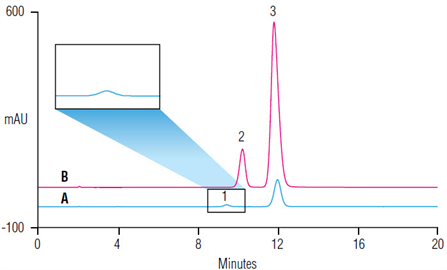
Risedronate is a pyridinyl bisphosphonate that is used as a drug for the prevention and treatment of osteoporosis. Risedronate poses analytical challenges for reversed-phase (RP) high-performance liquid chromatography (HPLC) due to the presence of two polar phosphonate groups. This study evaluates and validates a USP monograph method for risedronate analysis using ion chromatography.| Market: | Pharma |
| Keywords: | Actonel, API, AS7, Bisphosphonate, EDTA, IC, Ion Chromatography, Pharmaceutical, Pharmacopeia, risedronate, Risedronate Related Compound, USP monograph |
| Matrix: | Drug |
| Author: | Avanthi Kanmatareddy, Brian De Borba, and Jeff Rohrer |
| Affiliation: | Thermo Fisher Scientific |
For Research Use Only. Not for use in diagnostic procedures.