Parts List
Friend
Find methods for your needs
AN164: Assay for Citrate and Phosphate in PharmaceuticalFormulations Using Ion Chromatography
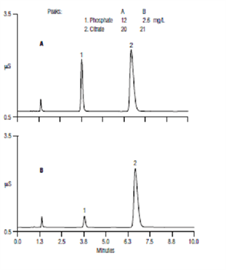
Description
In this application, we report on the validation of an IC method for the determination of phosphate and total citric acid in pharmaceutical formulations with a hydroxide-selective, anion-exchange column and suppressed conductivity detection. The method incorporates an electrolytic eluent generator to automatically produce a simple isocratic potassium hydroxide eluent, allowing the separation of phosphate and citrate on an Thermo Scientific Dionex IonPac AS11 column in less than 10 min.| Market: | Pharma |
| Keywords: | Pharmaceutical Dosage Forms, Thermo Scientific Dionex IonPac AS11 Column, USP, Citric acid, Citrate, phosphate, suppressed conductivity detection, Drug Product, <345>, GC 345, General Chapter |
| Matrix: | Pharmaceutical formulations |
| Author: | Brian De Borba and Jeff Rohrer |
| Affiliation: | Thermo Fisher Scientific |
For Research Use Only. Not for use in diagnostic procedures.
