Find methods for your needs
Refine by Feature
Displaying 1-4 of 4 results for Tag: Infliximab
High-precision, automated peptide mapping of proteins
Instrument Type: UHPLCThe biopharmaceutical industry continues to develop protein-based biotherapeutics in increasing numbers. Due to their complexity and biotechnological production, there are many attributes that need to be analyzed to guarantee their safety and efficacy. Peptide mapping is used to measure several critical quality attributes (CQA) required for the characterization of any biotherapeutic protein. The analysis is used to confirm that the correct sequence has been expressed for the protein and to check for post-translational and chemical modifications.
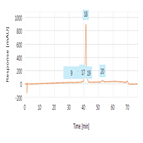
Separation of oxidized and deamidated impurities of Infliximab by reverse phase chromatography
Instrument Type: HPLC (Biocompatible)The Thermo Scientific Dionex UltiMate 3000 Bio-LC system is applied for the Reverse phase analysis of Infliximab. The separation was performed on a Thermo Scientific MabPac RP column with UV detection at 214 nm. All oxidized and deamidated impurities are well separated.
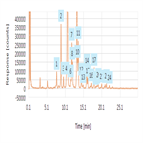
A high resolution separation of 2-AA derivatized N-Glycans from a commercial chimeric IgG1 monoclonal antibody (Infliximab)
Instrument Type: UHPLCThe Thermo Scientific Vanquish H UHPLC system was used for the high resolution determination of 2-AA (anthranilic acid) labelled N-glycans released from a commercial chimeric IgG1 mAb (Infliximab). The separation is performed on a 150 mm Accucore 150-Amide-HILIC column with fluorescence detection giving separation in less than 30 minutes.N-Glycans present at the asparagine 297 residue in the CH2 domain of the Fc region of IgG play a crucial role in modulating the structural stability and functional activity relationship of the antibody.
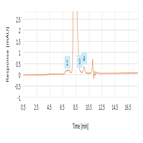
A universal chromatography method for aggregate analysis of monocolonal antibodies
Instrument Type: UHPLCAnalysis of protein aggregation of five important biotherapeutic monoclonal antibodies (mAbs) by size-exclusion chromatography, showing the universal applicability of the Thermo Scientific MAbPac SEC-1 column for aggregate analysis of mAbs. The biopharmaceutical industry continues to develop mAb-based biotherapeutics have several key quality attributes that require quantification. This shows the ability to determine quickly and accurately the percentage of aggregates and fragments for five therapeutics as shown with full details in the AN21601 download.