Find methods for your needs
Refine by Feature
Displaying 1-5 of 26 results for Tag: Syncronis C18
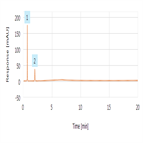
Related substances analysis of cefadroxyl monohydrate using a C18 HPLC column as per EP 8.0 monograph method
Instrument Type: HPLCThe Thermo Scientific Dionex UltiMate 3000 LC system is applied for the related substances analysis of cefadroxyl monohydrate. The analysis was performed on a Thermo Scientific Syncronis C18 HPLC column using the method described in the EP 8.0. The results obtained for resolution and signal-to-ratio met the criteria stated in the EP. The results obtained for resolution between the peaks due to EP Impurity A and EP Impurity B was 15.5 (EP criteria of minimum 5.0) and signal-to-ratio of EP Impurity B (0.4 µg/mL) was 56 (EP criteria of minimum 10). A sample chromatography is attached.
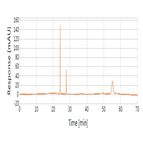
Related substances analysis of Cefaclor using a C18 HPLC column for EP 8.0 and JP XVI monograph method
Instrument Type: HPLCThe Thermo Scientific Dionex UltiMate 3000 LC system is applied for the related substances analysis of cefaclor. The analysis was performed on a Thermo Scientific Syncronis C18 HPLC column using the method described in the EP 8.0 and JP XVI. The results obtained for resolution and theoretical plates met the criteria stated in the EP/JP. The results obtained for resolution between the peaks due to cefaclor and EP Impurity D was 11.1 (EP criteria of minimum 2.0) and theoretical plates for cefaclor was 1,12,359 (JP criteria of minimum 40,000).
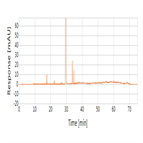
Organic impurities analysis of Cefotaxime using a C18 HPLC column following USP 39 monograph (procedure 2)
Instrument Type: HPLCThe Thermo Scientific Dionex UltiMate 3000 LC system is applied for the organic impurities (procedure 2) analysis of Cefotaxime Sodium. The analysis was performed on a Thermo Scientific Syncronis C18 HPLC column using the method described in the USP 39. The results obtained for resolution and tailing factor met the criteria stated in the USP. The results obtained for resolution between the peaks due to Cefotaxime and Cefotaxime related compound E was 13.6(USP criteria of minimum 4.0) and tailing factor for cefotaxime (10 µg/mL) was 1.0 (USP criteria of maximum 2).
Fast and reliable method for the analysis of testosterone, androstenedione, and 17-hydroxy progesterone from human plasma
Instrument Type: LCMSMSTo describe an accurate and precise high-throughput analytical technique for the analysis of testosterone, androstenedione, and 17-hydroxy progesterone utilizing micro-scale solid phase extraction (SPE), followed by liquid chromatography coupled with triple quadrupole mass spectrometry (LC-MS/MS). For research use only. Not for use in diagnostic procedures.
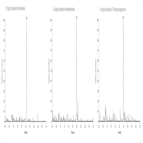
EP 8.0 monograph - impurity determination of ofloxacin using a C18 HPLC column
Instrument Type: HPLCThe Thermo Scientific Dionex UltiMate 3000 LC system is applied for the analysis of ofloxacin and its impurities. The separation was performed on a Thermo Scientific Syncronis C18 HPLC column using the method described in the EP 8.0 monograph. The results obtained for resolution exceeded the criteria stated in the EP. Resolution between impurity E and ofloxacin was 3.3 (EP criteria of not less than 2.0).