Find methods for your needs
Refine by Feature
Displaying 1-5 of 10 results for Tag: DI Water
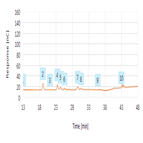
TN72264: HPAE-PAD N-linked Oligosaccharide Profiling of IgG
Instrument Type: ICThis technical note reports an HPAE-PAD method to profile IgG N-linked oligosaccharides and N-linked high-mannose type oligosaccharides that are atypical for human polyclonal IgG but sometimes found on mAbs. This HPAE-PAD method is orthogonal to other oligosaccharide methods applied to IgG. More information on the development and application of the separation described in the technical note can be found in Rohrer, et al. as referenced in the document.
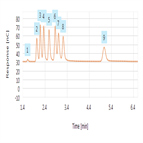
AN72210: Fast Determination of Biofuel Sugars by HPAE-PAD
Instrument Type: ICThis work updates the column used in AN1161 with a shorter 4 × 150 mm format column. The combination of the smaller resin particle size of the Dionex CarboPac SA10-4μm column and the shorter column used here results in separation of eight common sugars in less than six minutes. This is significant time savings as compared to the eight-minute runtime achieved in AN1089. The shorter run time allows for faster sample turnaround times and reduced eluent consumption, thereby improving the overall process economics. Results for method linearity, accuracy, and robustness are presented here.
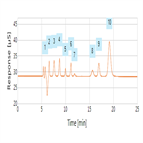
Dionex IonPac ICE AS6 Column Performance Test Using its QAR Method
Instrument Type: ICBefore running any samples, Thermo Scientific recommends that you first confirm the performance of the column by reproducing the lot validation report chromatogram shipped with column.Compare your results with the one reported in the quality assurance report. At least three injections should be made. This record provides an eWorkflow for executing the QAR method on an ICS-5000 system.<enter final 90 characters of description>
Dionex CarboPac PA210-Fast-4µm Column Performance Test Using its QAR Method
Instrument Type: ICBefore running any samples, Thermo Scientific recommends that you first confirm the performance of the column by reproducing the lot validation report chromatogram shipped with column. Compare your results with the one reported in the quality assurance report. At least three injections should be made. This record provides an eWorkflow for executing the QAR method on an ICS-5000+ system. An ICS-6000 can be used for this application.
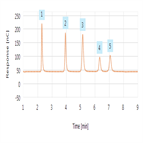
AN1144: Ion Chromatography Assay for Lithium in Lithium Hydroxide
Instrument Type: ICThe IC-based method described in this application note uses a Thermo Scientific Dionex IonPac CS16 cation-exchange column, an electrolytically generated MSA eluent, and suppressed conductivity detection to determine lithium in lithium hydroxide. The method proposed in this application note was validated following the guidelines outlined in USP General Chapter <1225>, Validation of Compendial Procedures to meet the requirements for lithium and calcium quantification prescribed in the lithium hydroxide USP monograph. An ICS-6000 can be used for this application.