Find methods for your needs
Refine by Feature
Displaying 1-4 of 4 results for Tag: Trimethoprim
Trace analysis of pharmaceuticals and organic contaminants in water
Instrument Type: LCMSMSWe present the reliable and accurate quantitative analysis of contaminants at the pg/mL level in drinking water using the Thermo Scientific EQuan MAX Plus LC-MS system coupled to the Thermo Scientific TSQ Endura triple quadrupole mass spectrometer. Excellent reproducibility was shown for the target compounds in tap water using 1 mL injections at 0.37× maximum effluent concentration.
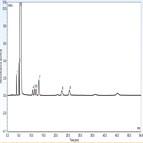
USP 38 monograph: impurity determination of trimethoprim using a C18 HPLC column
Instrument Type: HPLCThe Thermo Scientific Dionex UltiMate 3000 LC system is applied for the analysis of trimethoprim and its impurities. The separation was performed on a Thermo Scientific Acclaim 120 C18 HPLC column using the method described in the USP 38 monograph. The results obtained for resolution exceeded the criteria stated in the USP. Resolution between trimethoprim and diaveridine peak was 2.97 (USP criteria of not less than 2.5).
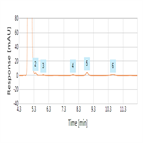
EP 8.0 monograph: impurity determination of trimethoprim (for impurities H and I) using a cyano HPLC column
Instrument Type: HPLCThe Thermo Scientific Dionex UltiMate 3000 LC system is applied for the analysis of trimethoprim and its impurities (H and I). The separation was performed on a Thermo Scientific Betasil CN HPLC column using the method described in the EP 8.0 monograph. The results obtained for resolution and relative retention time (RRT) exceeded the criteria stated in the EP. Resolution between impurity B and trimethoprim was 5.9 (EP criteria of not less than 2.0), RRT of impurity H was 1.8 (EP criteria of about 1.8).
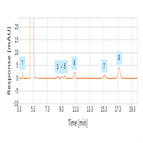
EP 8.0 monograph: impurity determination of trimethoprim (for impurities E, D, G, B, J and F) using a C18 HPLC column
Instrument Type: HPLCThe Thermo Scientific Dionex UltiMate 3000 LC system is applied for the analysis of trimethoprim and its impurities (E, D, G, B, J and F). The separation was performed on a Thermo Scientific Hypersil Gold HPLC column using the method described in the EP 8.0 monograph. The results obtained for resolution and tailing factor exceeded the criteria stated in the EP. Resolution between impurity E and trimethoprim was 7.7 (EP criteria of not less than 2.5) and tailing factor for trimethoprim was 1.1 (EP criteria not more than 2.0).