Find methods for your needs
Refine by Feature
Displaying 1-5 of 9 results for Tag: Syncronis aQ
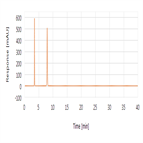
Organic impurities analysis of sulfacetamide sodium using a polar end-capped C18 HPLC column as per USP 39 monograph method
Instrument Type: HPLCThe Thermo Scientific Dionex UltiMate 3000 LC system is applied for the organic impurities analysis of Sulfacetamide Sodium. The analysis was performed on a Thermo Scientific Syncronis aQ HPLC column using the method described in the USP 39 monograph. The results obtained for resolution, tailing factor and %RSD met the criteria stated in the USP. The results obtained for resolution between sulfacetamide and sulfanilamide was 21 (USP criteria of NLT 5.0), tailing factor for sulfacetamide was 0.98 (USP criteria of NMT 1.5) and %RSD for sulfacetamide was 1.0% (USP criteria of NMT 2.0%).
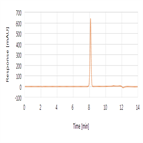
Assay analysis of Sulfacetamide sodium using a polar end-capped C18 HPLC column as per USP 39 monograph method
Instrument Type: HPLCThe Thermo Scientific Dionex UltiMate 3000 LC system is applied for assay analysis of sulfacetamide Sodium. The analysis was performed on a Thermo Scientific Syncronis aQ HPLC column using the method described in the USP 39 monograph. The results obtained for tailing factor and %RSD met the criteria stated in the USP. The results obtained for the tailing factor for sulfacetamide was 0.94 (USP criteria was 0.9 to 1.8) and %RSD for sulfacetamide was 0.1% (USP criteria was NMT 0.5%).
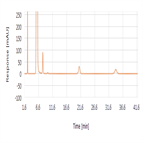
Related substances analysis of sulfacetamide sodium using a polar end-capped C18 HPLC column as per EP 8.0 monograph method
Instrument Type: HPLCThe Thermo Scientific Dionex UltiMate 3000 LC system is applied for the related substances analysis of Sulfacetamide Sodium. The analysis was performed on a Thermo Scientific Syncronis aQ HPLC column using the method described in the EP 8.0. The results obtained for resolution met the criteria stated in the EP. The results obtained for resolution between the peaks due to Impurity A and sulfacetamide was 14.5 (EP criteria of minimum 5.0).
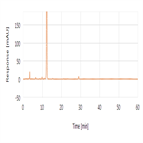
Related substances analysis of cefotaxime sodium using a polar end-capped C18 HPLC column as per EP 8.0 monograph method
Instrument Type: HPLCThe Thermo Scientific Dionex UltiMate 3000 LC system is applied for the related substances analysis of Cefotaxime Sodium. The analysis was performed on a Thermo Scientific Syncronis aQ HPLC column using the method described in the EP 8.0. The results obtained for resolution and symmetry factor met the criteria stated in the EP. The results obtained for resolution between the peaks due to Impurity E and Cefotaxime was 6.7 (EP criteria of minimum 3.5) and asymmetry factor for cefotaxime was 1.1 (EP criteria of maximum 2).
Assay and related substances analysis of Cefotaxime sodium using a C18 HPLC column as per JP XVI and KP X monograph methods
Instrument Type: HPLCThe Thermo Scientific Dionex UltiMate 3000 LC system is applied for the assay and related substances analysis of Cefotaxime Sodium. The analysis was performed on a Thermo Scientific Syncronis aQ HPLC column using the method described in the JP XVI/KP X. The results obtained for resolution and symmetry factor met the criteria stated in the JP/KP. The results obtained for resolution between the peaks due to desacetyl Cefotaxime and Cefotaxime was 32.8(JP/KP criteria of minimum 20.0) and symmetry factor for cefotaxime was 1.0 (JP/KP criteria of maximum 2).