Find methods for your needs
Refine by Feature
Displaying 1-3 of 3 results for Tag: JP XVI monograph
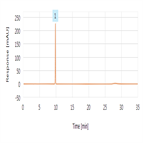
Related substances analysis of cefepime dihydrochloride hydrate using a C18 HPLC column as per JP XVI and KP X monograph method
Instrument Type: HPLCThe Thermo Scientific Dionex UltiMate 3000 LC system is applied for the related substances analysis of cefepime dihydrochloride hydrate. The analysis was performed on a Thermo Scientific Syncronis aQ HPLC column using the method described in the JP XVI and KP X. The results obtained for theoretical plates and %RSD for cefepime met the criteria stated in the JP/KP monograph. The results obtained for theoretical plates of cefepime was 54,132 (JP/KP criteria of not less than 6,000) and %RSD for cefepime was 0.1% (JP/KP criteria of not more than 2).
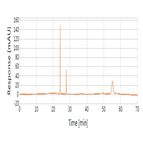
Related substances analysis of Cefaclor using a C18 HPLC column for EP 8.0 and JP XVI monograph method
Instrument Type: HPLCThe Thermo Scientific Dionex UltiMate 3000 LC system is applied for the related substances analysis of cefaclor. The analysis was performed on a Thermo Scientific Syncronis C18 HPLC column using the method described in the EP 8.0 and JP XVI. The results obtained for resolution and theoretical plates met the criteria stated in the EP/JP. The results obtained for resolution between the peaks due to cefaclor and EP Impurity D was 11.1 (EP criteria of minimum 2.0) and theoretical plates for cefaclor was 1,12,359 (JP criteria of minimum 40,000).
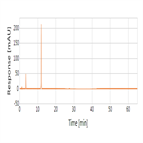
Assay and related substances analysis of Cefotaxime sodium using a C18 HPLC column as per JP XVI and KP X monograph methods
Instrument Type: HPLCThe Thermo Scientific Dionex UltiMate 3000 LC system is applied for the assay and related substances analysis of Cefotaxime Sodium. The analysis was performed on a Thermo Scientific Syncronis aQ HPLC column using the method described in the JP XVI/KP X. The results obtained for resolution and symmetry factor met the criteria stated in the JP/KP. The results obtained for resolution between the peaks due to desacetyl Cefotaxime and Cefotaxime was 32.8(JP/KP criteria of minimum 20.0) and symmetry factor for cefotaxime was 1.0 (JP/KP criteria of maximum 2).