Find methods for your needs
Refine by Feature
Displaying 1-3 of 3 results for Tag: Cefradine
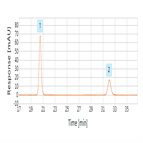
EP 8.0 monograph: impurity determination of cefradine using reversed-phase HPLC-UV
Instrument Type: HPLCThe Thermo Scientific Dionex UltiMate 3000 LC system is applied for the analysis of Cefradine and its impurities. The separation was performed on a Thermo Scientific Hypersil Gold column using the method described in the EP 8.0 monograph. Resolution between cefalexin and cefradine was 7.3 (EP criteria of minimum 4.0). Retention time of cefradine was 15.1 minutes (EP criteria of about 15 minutes). The criteria for relative retention time were also exceeded.
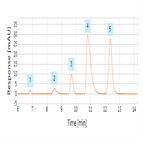
EP 8.0 monograph - assay analysis of cefalexin using reversed-phase HPLC-UV
Instrument Type: HPLCThe Thermo Scientific Dionex UltiMate 3000 LC system is applied for the assay analysis of cefalexin. The separation was performed on a Thermo Scientific Acclaim 120 C18 HPLC column using the method described in the EP 8.0 monograph. The results obtained for resolution exceeded the criteria stated in the EP. Resolution between cefalexin and cefradine was 14.1 (EP criteria of minimum of 4.0).
EP 8.0 monograph - impurity determination of ampicillin using a C18 HPLC column
Instrument Type: HPLCThe Thermo Scientific Dionex UltiMate 3000 LC system is applied for the analysis of ampicillin and its impurities. The separation was performed on a Thermo Scientific Acclaim 120 C18 HPLC column using the method described in the EP 8.0 monograph. The results obtained for resolution and relative retention time (RRT) exceeded the criteria stated in the EP. Resolution between ampicillin and cefradine was 3.23 (EP criteria minimum of 3.0). RRT with reference to ampicillin and ampicillin dimer in reference solution (d) was 2.84 (EP criteria about 2.8).