Find methods for your needs
Refine by Feature
Displaying 1-3 of 3 results for Tag: Silver Cyanide
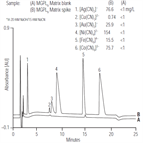
AU149: Determination of Metal Cyanide Complexes in Solid Wastes by Anion-Exchange Chromatography with UV Absorbance Detection
Instrument Type: ICThis application update describes the determination of the metal cyanide complexes of iron, cobalt, silver, gold, copper, and nickel in solid wastes by anion-exchange chromatography with UV absorbance detection. Metal cyanide complexes are solubilized and recovered by an alkaline extraction procedure (SW846 Method 9013) prior to chromatographic analysis. Two analytical approaches are available depending on the concentration of metal cyanides expected in the leachate; the two methods differ only in how the sample is injected.
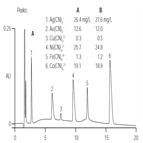
AU147: Direct Determination of Metal Cyanides by Ion Chromatography with UV Absorbance Detection
Instrument Type: ICMetal cyanide complexes are of environmental concern because they release cyanide upon dissociation. The metal cyanide complexes of silver, gold, copper, nickel, iron, and cobalt are separated on an IonPac AS11 column and quantified by measuring their absorbance at 215 nm. Use of the 2-mm AG11/AS11 column set provides different selectivity, lower eluent use, and better solvent compatibility than the AG5/AS5 column set featured in AN 55. The method was evaluated for reproducibility, linearity, accuracy, precision, and spike recovery from various matrices.
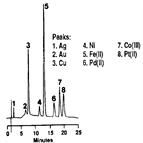
AN55: Determination of Metal Cyanides
Instrument Type: ICTransition metal cyanides typically exist in solution as the anionic cyanometallates, M (CN)x^n-. Since these complexes are very stable (formation constants being as high as 10^35 for Fe (CN)6^4-), they may be separated by anion exchange chromatography. Cyano complexes of most of the transition metals absorb low-wavelength ultraviolet light, making detection at 215 nm a convenient method of determination. Also refer to AN161 and AU147 for an updated method.