Find methods for your needs
Refine by Feature
Displaying 1-2 of 2 results for Tag: Chondroitin Sulfate
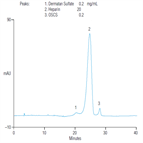
AU178: A Faster Solution with Increased Resolution for Determining Chromatographic Identity and Absence of OSCS in Heparin Sodium
Instrument Type: ICHere, an improved method for resolving DS and OSCS in heparin with an IonPac AS11-HC (2 × 250 mm) column is demonstrated, which is similar to the US FDA method previously described. AU178 updates Application Note 235. The higher capacity of the IonPac AS11-HC column, relative to the IonPac AS11 column described in the current USP method, minimizes the possibility of column overload and provides improved resolution between DS and heparin. The microbore column format reduces eluent consumption and hence the time and labor required for manual eluent preparation.
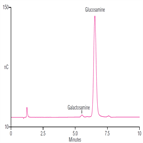
AN233: Determination of Galactosamine Containing Organic Impurities in Heparin by HPAE-PAD
Instrument Type: ICIn this application note, the organic impurities in heparin are determined by the HPAE-PAD method using the CarboPac PA20 column following the USP monograph method. This method was repeated using manually prepared eluents and an electrolytically generated eluent, with both eluent preparation options providing data that exceeds the system suitability requirements in the monograph.