Find methods for your needs
Refine by Feature
Displaying 1-4 of 4 results for Tag: Amoxicillin
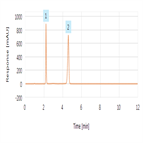
Assay analysis of Amoxicillin and Clavulanate Potassium as per USP
Instrument Type: HPLCThe Thermo Scientific Dionex UltiMate 3000 LC system is applied for the analysis of Amoxicillin and Clavulanate Assay as per USP. The analysis was performed on a Thermo Scientific Syncronis aQ L1 HPLC column using the method described in the USP 40 monograph. The results obtained were found to be within acceptance criteria as stated in the USP. Resolution between Amoxicillin and clavulanic acid was 14.0 (USP criteria: not less than 3.5). Tailing Factors observed were 0.84 & 1.25 respectively (USP criteria: not more than 1.5 for each analyte). RSD found to be less than 2% for both analytes
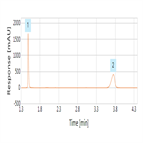
USP 38 monograph - impurity determination of ampicillin using a C18 selectivity HPLC column
Instrument Type: HPLCThe Thermo Scientific Dionex UltiMate 3000 LC system is applied for the analysis of ampicillin and its impurities. The separation was performed on a Thermo Scientific Hypersil Gold HPLC column using the method described in the USP 38 monograph. All USP criteria was exceeded. Resolution between amoxicillin and ampicillin was 22 (USP criteria of not less than 10). Signal to noise ratio was 140 (USP criteria not less than 3). Tailing factor of ampicillin was 0.8 (USP criteria not more than 1.4) and relative standard deviation for standard solution was 0.04% (USP criteria not more than 10.0%).
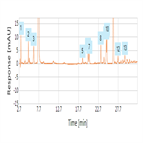
EP 8.0 monograph: impurity determination of amoxicillin using a C18 HPLC column
Instrument Type: HPLCThe Thermo Scientific Dionex UltiMate 3000 LC system is applied for the analysis of amoxicillin and its impurities. The separation was performed on a Thermo Scientific Hypersil Gold HPLC column using the method described in the EP 8.0 monograph. The results obtained for resolution and relative retention time (RRT) exceeded the criteria stated in the EP. Resolution between amoxicillin and cefadroxil was 10.0 (EP criteria of not less than 2.0). RRT of impurity C and impurity J with respect to amoxicillin was 3.2 and 3.8 respectively (EP criteria of impurity C 3.4 and impurity J 4.1).
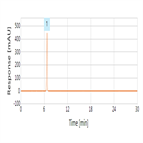
Amoxicillin capsules assay according to the Chinese Pharmacopoeia using a Thermo Scientific Acclaim 120 C18 HPLC column
Instrument Type: HPLCThe Thermo Scientific HPLC system is applied for the analysis of amoxicillin capsules according to the Chinese Pharmacopoeia method. The separation is performed on a Thermo Scientific Acclaim 120 C18 HPLC column, with UV detection at 254 nm.