Find methods for your needs
Refine by Feature
Displaying 1-2 of 2 results for Tag: Hydrochlorothiazide
EP 8.0 monograph: impurity determination of hydrochlorothiazide using a C18 selectivity HPLC column
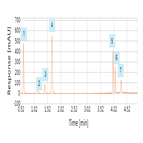
Instrument Type: HPLCThe separation was performed on a Thermo Scientific Hypersil Gold column using the method described in the EP 8.0 monograph. The results obtained for resolution and relative retention time (RRT) exceeded the criteria stated in the EP. Resolution between Hydrochlorothiazide and impurity A was 5.7 (EP criteria of not less than 2.5). RRT of impurity A, B and C with reference to Hydrochlorothiazide was 0.9 for impurity A, 0.7 for impurity B and 2.9 for Impurity C (EP criteria about 0.9, 0.7 and 2.8 respectively for impurity A, B and C).
Reproducible separation of acidic and neutral drugs using a solid core C18 HPLC column
Instrument Type: HPLCThe separation of acidic and neutral drugs was successfully achieved on the Thermo Scientific Accucore C18 column, providing an excellent choice for the analysis of these drugs giving excellent retention time reproducibility and resolution.