Parts List
Friend
Find methods for your needs
Analysis of pharmaceutical products for their elemental impurities with the Thermo Scientific iCAP RQ ICP-MS
Description
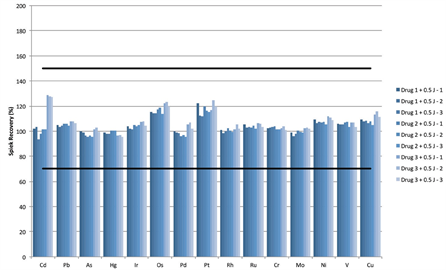
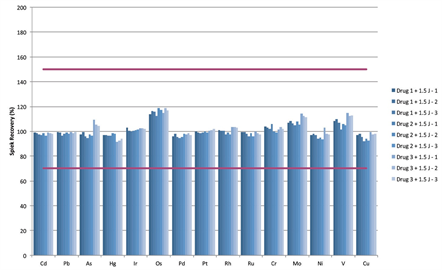
To demonstrate the use of the Thermo Scientific™ iCAP™ RQ ICP-MS to accurately determine concentrations of elemental impurities in pharmaceutical products brought into solution using microwave digestion. All sample preparation, measurement and data evaluation to be compatible with the guidelines defined in USP chapters <232> Elemental Impurities – Limits and <233> Elemental Impurities Procedures.| Market: | Pharma |
| Keywords: | USP 232, Microwave digestion, United States Pharmacopeia, FDA 21 CFR part 11, Pharmaceutical compliance, Pharmaceutical preparations, USP 233 |
| Matrix: | 1.2% HNO3 and 0.5% HCl |
| Author: | Julian Wills and Daniel Kutscher |
| Affiliation: | Thermo Fisher Scientific, Bremen, Germany |
For Research Use Only. Not for use in diagnostic procedures.